ulti med Products (Deutschland) GmbH specialises in the development, manufacture as well as worldwide export of high-quality In-vitro diagnostics and drug tests. The high quality standards applicable to our products and our quality management system are confirmed by an EN ISO certification. All products stand out due to their simple handling and verified reliability.
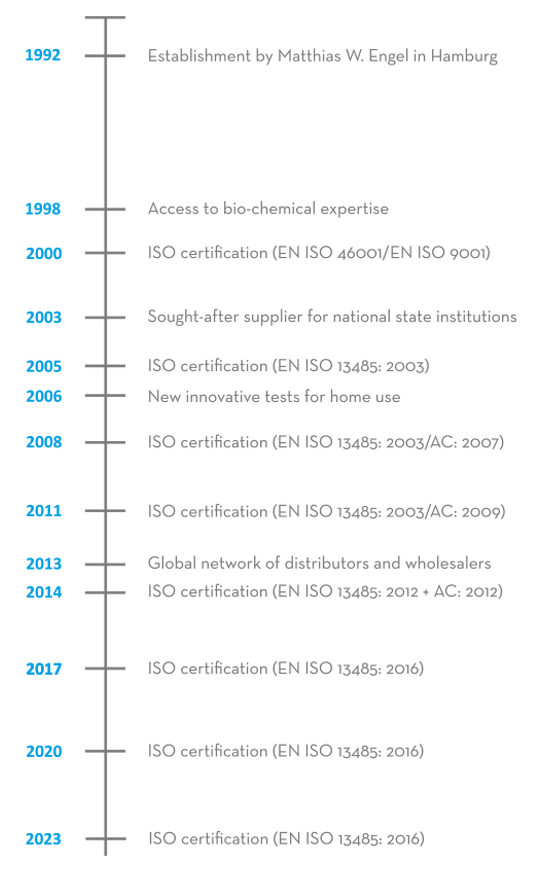
The ULTIMATE Pharma Products Import and Export GmbH was founded in 1992 by Matthias W. Engel. The company sold dietary supplements as well as items for use in surgeries and hospitals to markets in the EU and worldwide through pharmaceutical wholesale traders.
In 1998, an expansion into the area of In-vitro diagnostics was initiated, which involved the recruitment of specialist personnel with bio-chemical expertise and which is still ongoing. This enabled the company to analyse antibodies, which provide the basis for manufacturing many In-vitro diagnostic products. Since then, the company has been able to utilise the acquired manufacturing processes to file numerous patent applications.
In 2000, the company obtained an ISO certification and changed its name to ulti med Products (Deutschland) GmbH. This meant that ulti med Products (Deutschland) GmbH was one of Germany’s first companies certified to EN ISO 46001/EN ISO 9001 for the manufacture of high-quality products In-vitro diagnostic. With this core competency, the company developed into an expert in the manufacture of products in this sector.
The continuously updated ISO quality standards and a large number of new developments in the area of In-vitro diagnostics, particularly for drug detection, meant that the company also became sought-after as a supplier to national state institutions, for instance from the justice system, the police and the military, from 2003 onwards. This allowed ulti med Products (Deutschland) GmbH to expand into a security-relevant market, where In-vitro diagnostics and drug testing play an important role.
In 2005, the company decided to make In-vitro diagnostics, which previously only had professional applications, available to private users as well. In 2006, having fulfilled all statutory requirements, ulti med Products (Deutschland) GmbH was able to launch the first of these new innovative tests for home use, thereby responding to special needs of private users.
With its global network of distributors and wholesalers, the company has been renowned since 2013 as an expert in the manufacture of products for In-vitro diagnostic and for drug screening in connection with various indications and in diverse areas of application. High product quality and regular new developments make ulti med Products (Deutschland) GmbH a reliable, globally operating partner for public institutions of the healthcare system, for public authorities as well as doctors’, surgeries, hospitals and private users.







