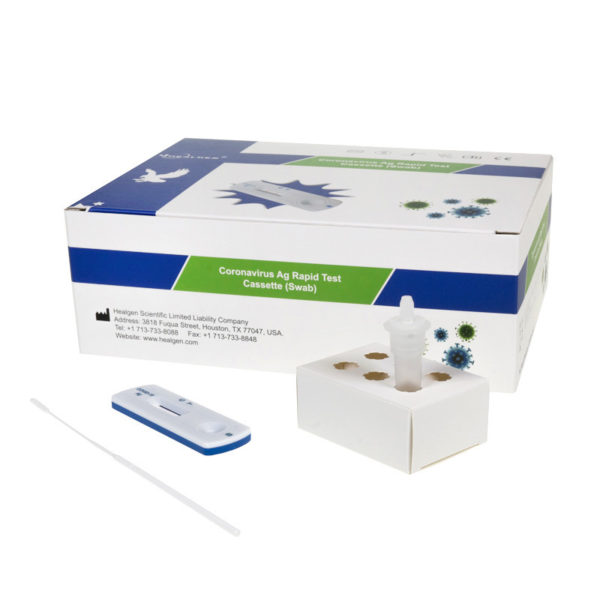
professional Healgen Corona Test
- Article ID
- GCCOV-502a/2
- manufacturer
- Healgen Scientific Limited
- Handling time
Product description
The Coronavirus Ag Rapid Test Cassette (Swab) is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) swab or nasal swab specimens directly from individuals who are suspected of COVID-19 by their healthcare provider within the first ten days of symptom onset, and asymptomatic individuals. It is intended to aid in the rapid diagnosis of SARS-CoV-2 infections. Negative results from patients with symptom onset beyond ten days should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed. The Coronavirus Ag Rapid Test Cassette (Swab) does not differentiate between SARS-CoV and SARS-CoV-2. Coronavirus Ag Rapid Test Cassette (Swab) is intended for use by healthcare professionals or trained operators who are proficient in performing rapid tests and trained clinical laboratory personnel specifically instructed on in vitro diagnostic procedures and proper infection control procedures or individuals similarly trained in point of care settings.
Summary and Explanation
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue, and dry cough. Nasal congestion, runny nose, sore throat, myalgia, and diarrhea are found in a few cases. This test is for detection of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively. To effectively monitor the SARS-CoV-2 pandemic, systematic screening and detection of both clinical and asymptomatic COVID-19 cases is critical. Particularly, the identification of subclinical or asymptomatic cases is important to reduce or stop the infection because these individuals may transmit the virus. Coronavirus Ag Rapid Test Cassette (Swab) allows effective screening of COVID-19 infection.
Principle of the test
The Coronavirus Ag Rapid Test Cassette (Swab) is an immunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to detect nucleocapsid protein from SARS-CoV-2 in direct nasopharyngeal (NP) swab or nasal swab. The test strip is composed of the following parts: namely sample pad, reagent pad, reaction membrane, and absorbing pad. The reagent pad contains the colloidal-gold conjugated with the monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2; the reaction membrane contains the secondary antibodies for nucleocapsid protein of SARS-CoV-2. The whole strip is fixed inside a plastic device. When the sample is added into the sample well, conjugates dried in the reagent pad are dissolved and migrate along with the sample. If SARS-CoV-2 nucleocapsid antigen is present in the sample, a complex forms between the anti-SARS-2 conjugate and the virus will be captured by the specific anti-SARS-2 monoclonal antibodies coated on the test line region (T). Absence of the test line (T) suggests a negative result. To serve as a procedural control, a red line will always appear in the control line region (C) indicating that proper volume of sample has been added and membrane wicking has occurred.